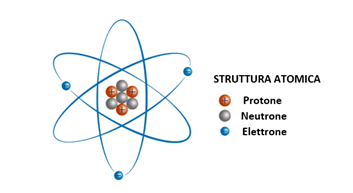
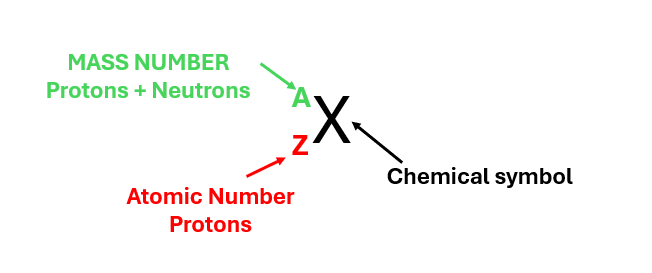
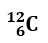An ion is a chemical species with a net electrical charge. It can be composed of a single atom (monoatomic ion) or a group of atoms (polyatomic ion).
When the net charge is positive, the ion is called an ANION. In the presence of a net positive charge, the ion is called a CATION.
Example:
- Na+; Cl–; Ca2+; O2- (monoatomic ions).
- NH4+, VO2+, CO32-, SO42-, PO43- (polyatomic ions).
CATIONS
Let’s focus on the following monoatomic cation:

The positive charge +1 indicates that the number of protons exceeds the number of electrons by one.
This is obtained by subtracting one electron from the neutral atom, NOT by adding one proton.
Li+ 3 protons; 2 electrons.
From the current information, it’s not possible to determine the number of neutrons.
(If the calculation of subatomic particles is unclear, click here).
A positive ion, obtained by the removal of one or more electrons, is called a CATION
ANIONS
Let’s focus on the anion F– obtained by the following neutral element:

Fluorine is composed of 9 protons, 10 neutrons, 9 electrons. The addition of one electron, results in the anion F– .
F– 9 protons; 10 neutrons 10 electrons
A negative ion, obtained by the addition of one or more electons, is called an anion.
A mistake that must be absolutely avoided, is adding or subtracting a proton instead of an electron. This mistake results in a change of the atomic number.
KEY POINTS:
- A monoatomic cation is a positive ion, composed of a single atom, obtained by the removal of one or more electrons from a neutral element.
- A monoatomic anion is a negative ion, composed of a single atom, obtained by the addition of one or more electrons from a neutral element.
SOLVED EXERCISE:
Calculate the number of protons, neutrons and electrons of the ion S2- from the following neutral atom:
The atomic structure of the neutral atom is composed of 16 protons, 16 neutrons e 16 electrons;
It’s necessary to add or subtract a number of electrons consistent with the charge of the ion;
Therefore, the structure of the ion is made up of di 16 protons, 16 neutrons e 18 electrons.